V V To W W

Used where the weight of each chemical is used and not the volume e g.
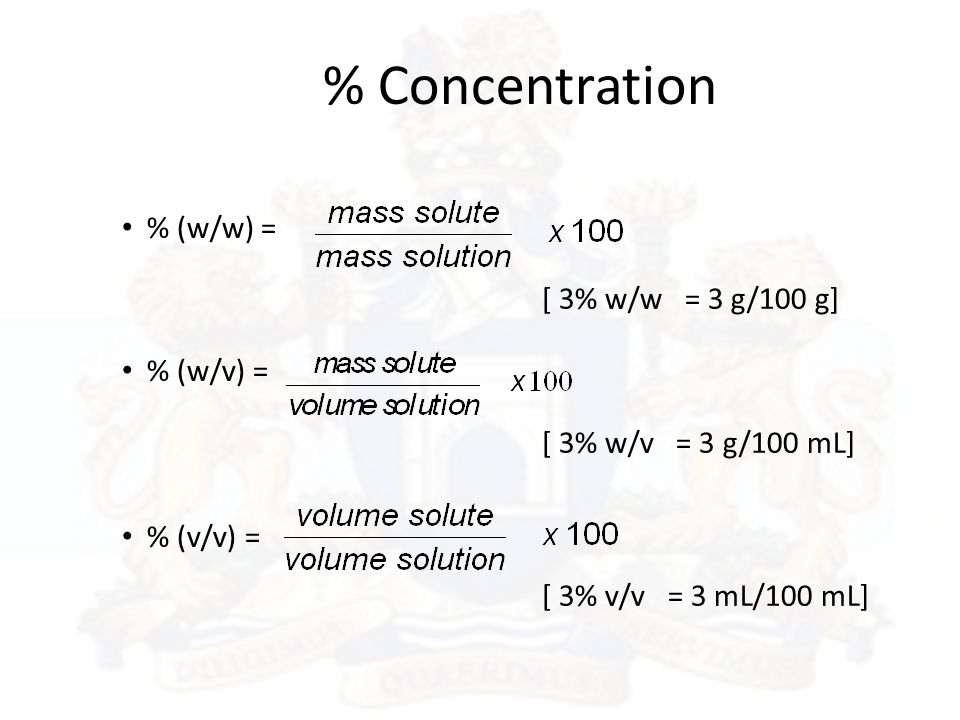
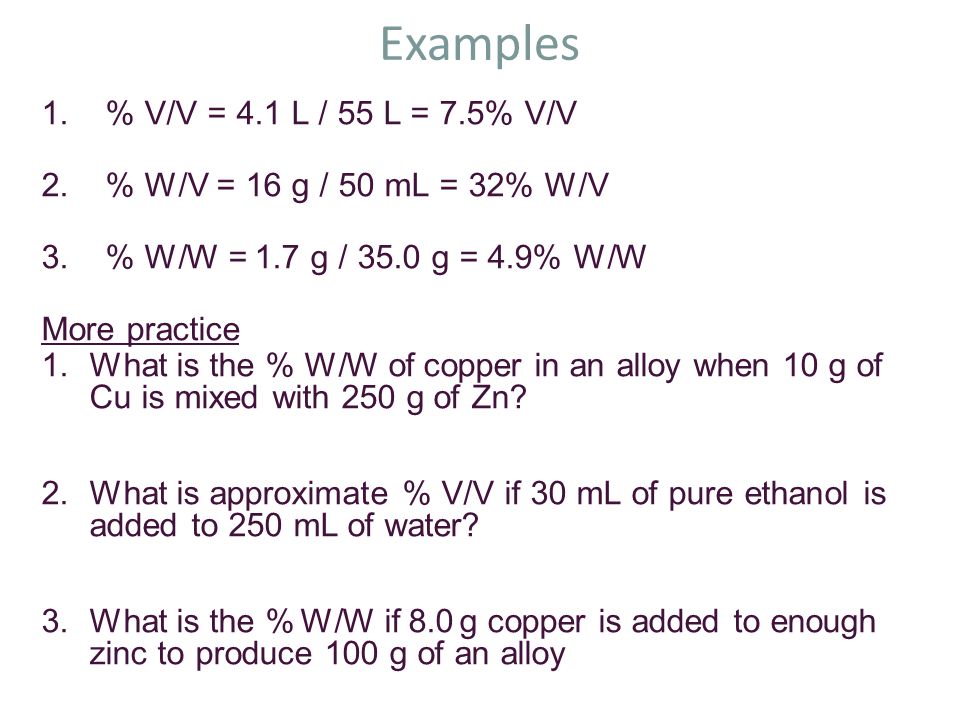
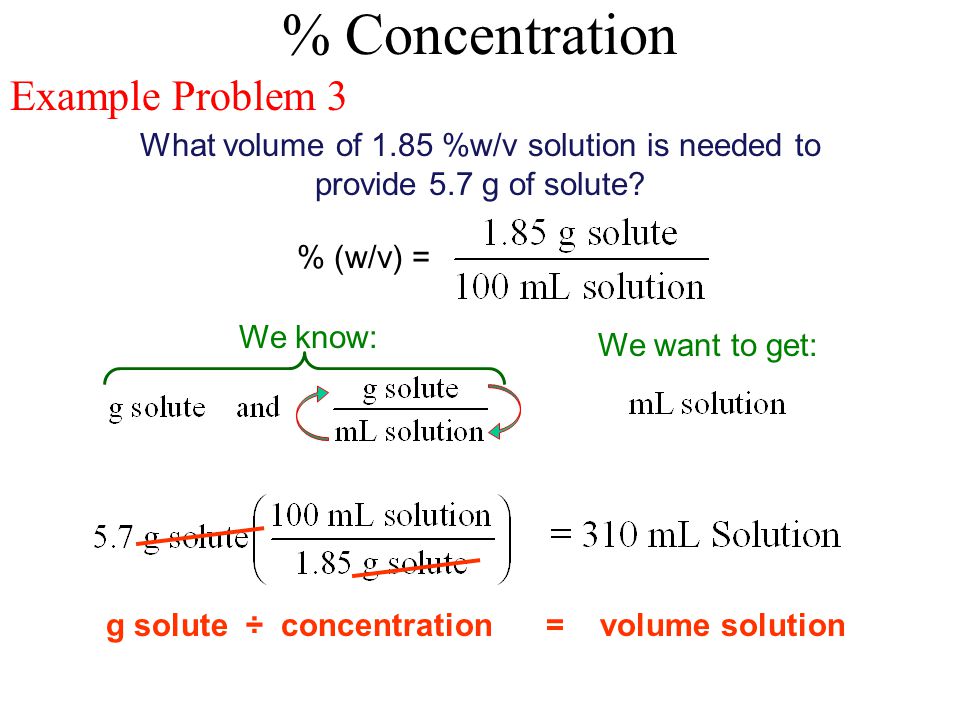
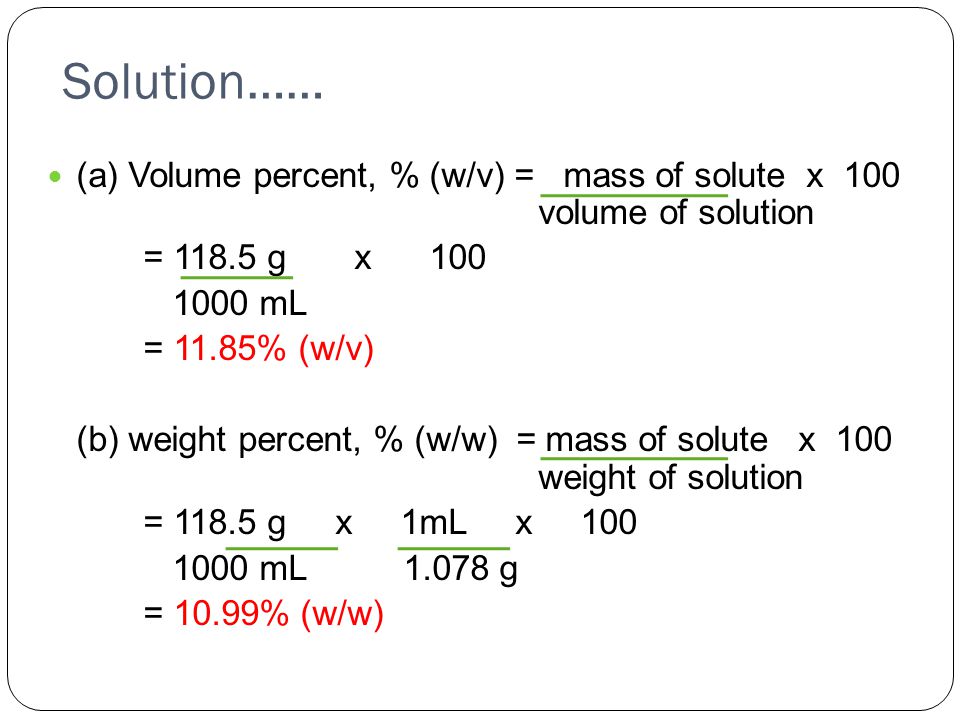
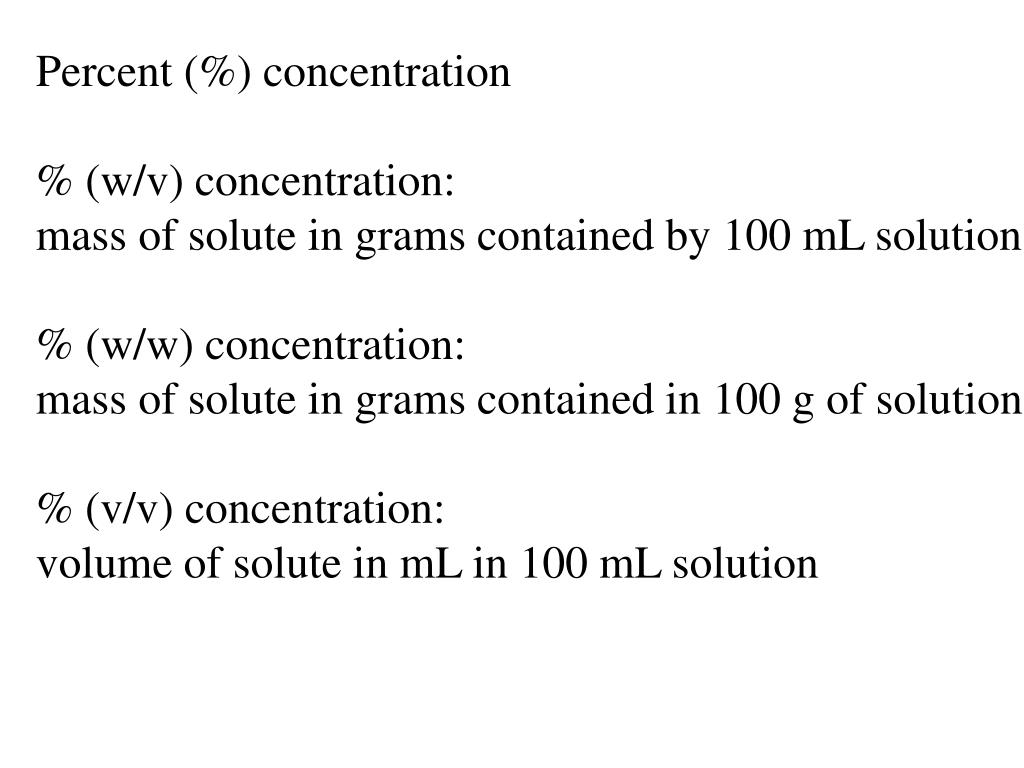
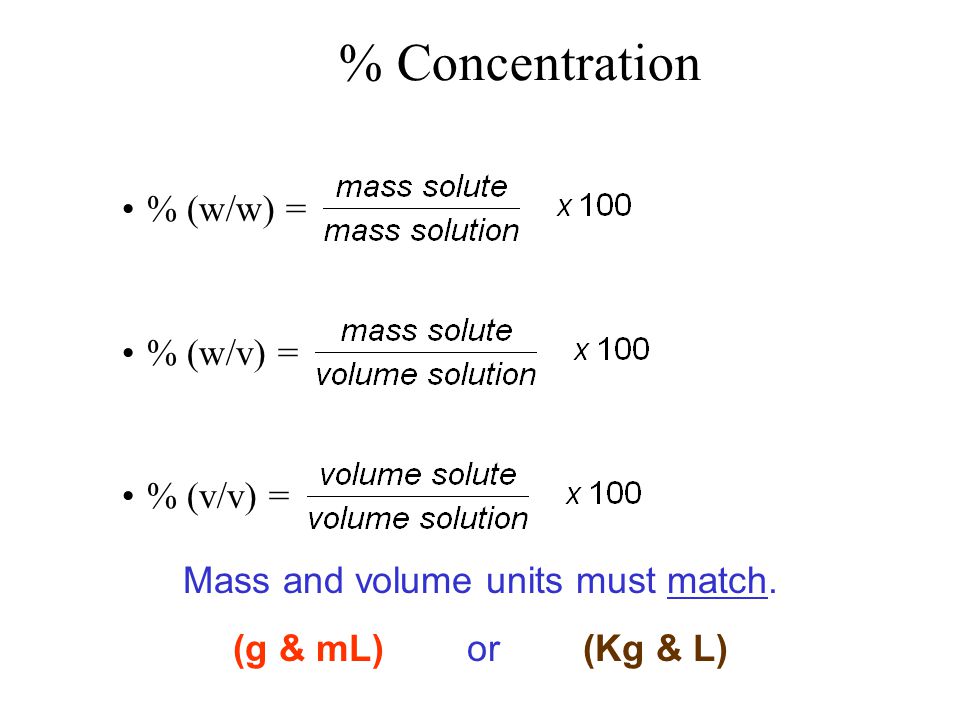
V v to w w. Used where a solid chemical is dissolved in liquid e g. Percent solutions can take the form of weight volume wt vol or w v weight weight wt wt or w w or volume volume vol vol or v v. Weight by volume percent w v tells you the mass of solute in grams that has been added to a 100 ml solution. For example a 50 v v solution would be 50 of one solution and 50 of another equal amounts of each in terms of volume.
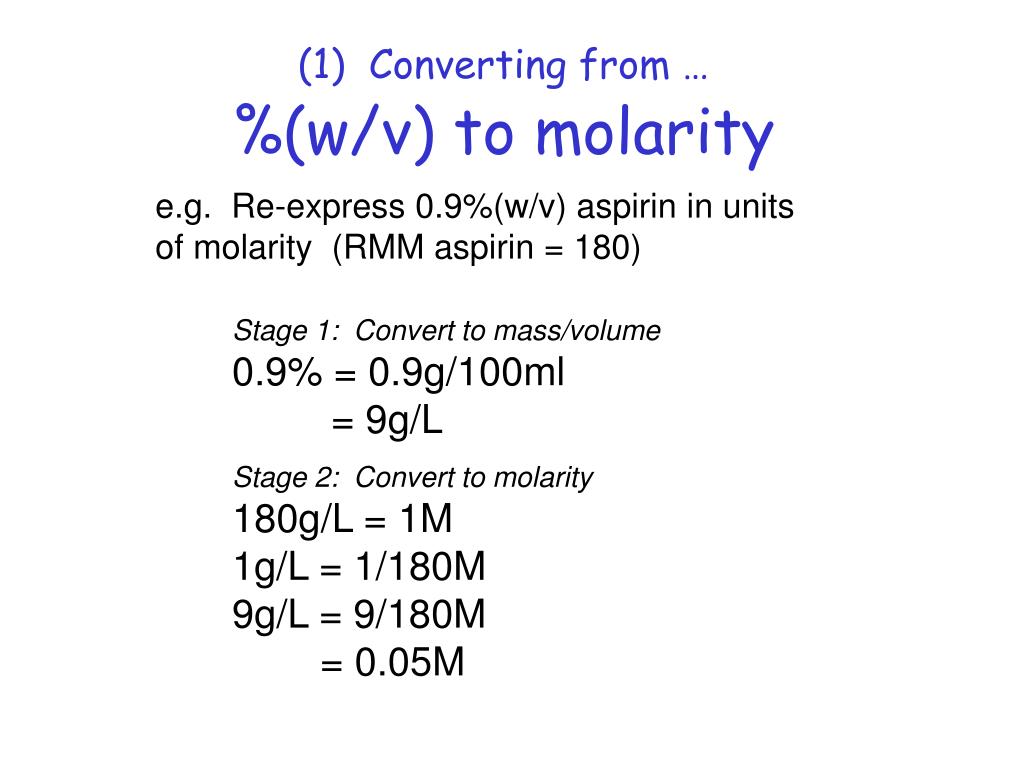
Therefore to figure out the w v of a 100ml solution that is made up of 65g nitric acid we would divide 65g by 100ml and then multiply the answer by 100. In each case the percentage concentration is calculated as the fraction of the weight or volume of the solute related to the total weight or volume of the solution. This tells us that there is a nitric acid solution of 65 w v. To convert v v to w w you have to multiply by the density of the solute and divide by the density of the solution.
If i dissolve 10 g of table salt sodium chloride to make up a total volume of 100 ml of solution then i have made a 10 w v solution of sodium chloride w w weight per weight. Some of the most common include molarity weight by volume volume by volume and weight by weight. There are many different ways of expressing the concentration of a given solution. When working out the v v of a solution the same method is used except it is the volume of the solute ml that is divided.
Unfortunately the solution is a mixture and the density is a function of the concentration. If the solution is very dilute you can assume the density of the solvent but generally you need a table of concentrative properties.